LABORATORY ETIQUETTE

Laboratory is a particular place, where students acquire practical knowledge with the help of hard ware and soft ware. Discipline, Punctuality, Sincerity, and carefulness are prerequisites for good performance in the laboratory.
Following rules and regulations are to be maintain by all students for good performance:
1. We should be punctual classes so that we can listen the instruction given to us. We should be attentive and serious in our classes.
2. Loose dresses like sharia’s, Punjabi and half shoe (slipper ) should be avid ed.
3. We must wear white coat (apron) and should have watch in the class.
4. Noise and confusion must be avoided in the laboratory.
5. Before starting and experiment we must have a theoretical background about the experiment.
6. When working in a group, we should participate equally in the experiment with the perfect understanding and proper co-operation.
7. We should try to learn the technical knowledge from the laboratory technician and teacher.
8. Before leaving the laboratory we should hand over the apparatus, equipment’s, Specimen, Regent to the laboratory technician.
9. Before leaving the laboratory every one should clean his/her own experiment table.
10. We must maintain a laboratory note book and should bring it to laboratory so that it may be signed regularly by the teacher.
Following rules and regulations are to be maintain by all students for good performance:
1. We should be punctual classes so that we can listen the instruction given to us. We should be attentive and serious in our classes.
2. Loose dresses like sharia’s, Punjabi and half shoe (slipper ) should be avid ed.
3. We must wear white coat (apron) and should have watch in the class.
4. Noise and confusion must be avoided in the laboratory.
5. Before starting and experiment we must have a theoretical background about the experiment.
6. When working in a group, we should participate equally in the experiment with the perfect understanding and proper co-operation.
7. We should try to learn the technical knowledge from the laboratory technician and teacher.
8. Before leaving the laboratory we should hand over the apparatus, equipment’s, Specimen, Regent to the laboratory technician.
9. Before leaving the laboratory every one should clean his/her own experiment table.
10. We must maintain a laboratory note book and should bring it to laboratory so that it may be signed regularly by the teacher.
COMMON LABORATORY GLASS WARES AND EQUIPMENTS
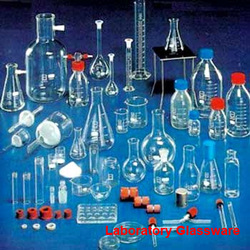
Laboratory glassware’s for biochemical use must be free from all forms of continuation. After using glass wares. It should be washed by.
Warm water containing soap detergent : 1% solution of detergent
Cleaning solution : it is mast efficient and composed of –
Potassium dichromate (k2cr2o7) : 10 gm
Concentrated (h2s04) : 25 ml
H2O : 75 ml
Those are mixed well and used for cleaning purpose as it is an oxidizing agent so it should be handled with extra care.
Different type of glassware's :
1.Pipette : it is a narrow glass tube with both ends open for transferring and measuring liquids by sucking them into the tube. The tip of the pipette is usually tapered.
Types : usually there are two types:
a. Graduated pipette.
b. Volumetric pipette.
Special type:a.Micro pipette or capillary pipette.
b.Van-slake pipette.
c.Automated pipette.
Usual size : 1ml, 2ml, 5ml, 10ml.
Use : For measurement of fluid, Reagent, Blood, Serum
2.Barrette : Barrette is a special hallow glass tube usually with a stopcock at the lower end. It is marked so that the amount of liquid released through the outlet will be known.
Usual size : 5-50 ml capacity. Micro Barrette have a maximum capacity of 2ml or less.
Use : a.in titration.
b.Any volume of liquid up to a certain limit may be out taken out with great accuracy.
3.Measuring cylinder : it is a long cylindrical piece of glass ware with calibration marking on it in on ascending order (zero at the button) a lip to facility pourcing liquid.
Usual size : measurement of relatively large volumes of liquid’s when accuracy is not essential.
Use : measurement of relatively large volumes of liquids when accuracy is not essential.
4.Flasks : a flask is a small bottle with a narrow neck.
Type :a.Volumetric flask
b.Conical flask
c.Rounded bottom flask
d.Flat bottom rounded
a.Volumetric flask : it is a pear-shaped, flat bottomed bottle made of high quality glass. It has a narrow long neck filled with ground glass and a single calibration mark.
Usual size : 50ml, 100ml, 500ml and 1000ml capacity.
Use : For titration
For boiling solutions when it is necessary to give evaporation to a minimum.
5.Beaker: beaker is a glass vessel with wide mouth. It is usually made of heat resistance glass.
Usual size : 5-500ml
Use : a.For weighting reagents in the preparation of solution.
b.For boiling of water and liquid.
c.Graduated beakers is used for estimating the volume of fluid.
6.Funnel : it is a conical, wide-open mouthed device for pouring through its open tube at end into another vessel.
Type : a.Filter funnel.
b.Separating funnel.
c.Filter funnel : it has a conical head and long narrow tube.
Use : to pour liquid’s into container.o To support filter paper during filtration.
b.Separating funnel : it is a special funnel, usually pear shaped or cylindrical. It has fitted ground glass stopper at the top and a plastic stop cock at the button.
Use : To separate two immiscible fluids. Eg, ether and water.
7.Reagent bottle : it is a glass container, has many shapes the usual shape is cylindrical with a narrow neck. It may be clean or coloured and upper end fitted with ground glass.
Usual size : 25-1000ml.
Use : for holding reagent, solution’s, specimen and media.
8.Test tubes : these are tube link containers with one open end.
Usual size : a.6 inch x 5/8 inch
b.20 ml capacity
Use :a.To carry out tests like biochemical, serological.
b.Test tubs are used to heat liquid.
c.Preserve sample like blood, urine, CSF etc.
9.Test tube holder : it has a catch and a handle.
Use : to hold test tube during heating.
10.Test tube rack : these are made of metal, plastic.
Use : to hold the test tube in upright position.
11.Syringe : a.Syringe has the following part- barel is provided with a needle.
b.Plunger which can be moved forwards and back wards.
Usual size : 3ml, 5ml, 10ml, 20ml and 50ml.
Use : to draw blood from blood easels.
To inject drugs.
12.Evaporating basin : it is an open, bowl like shallow container made of porcelain or heat resistant glass.
Use : to evaporate solutions to dryness.
13.Gas burner : to health solution, reagent etc.
14.Barrette stand : to hold barette.
Different types of laboratory equipments (apparatus) :
1.centrifuge Machine:
principal : when centrifugal force exceeds the force of gravity the heavier substances are thrown to the bottom of the centrifuge tube.
Use : to hasten the deposition of substance suspended in a liquid. The suspended matter are deposed, in order of weight, the heaviest one is settled first.
2.photoelectric calorimeter : it is an instrument that measure the intensity of color of a solution by registering the amount of a light observed by a given solution.
Parts : this instrument consists of –
a.source of light.
b.Monochromatic light and filter.
c.Curettage or tubes.
d.Photosensitive elements.
e.A sensitive galvanometer.
Calorimeter is based upon two law –
a.Lambert law
b.beer’s law.
Use : a.to measure concentration of substances such as glucose, cholesterol, electrolytes (na+, k+, ca+), enzymes (ast, alt), haemoglobin etc.
b.to measure activity of enzyme.
Warm water containing soap detergent : 1% solution of detergent
Cleaning solution : it is mast efficient and composed of –
Potassium dichromate (k2cr2o7) : 10 gm
Concentrated (h2s04) : 25 ml
H2O : 75 ml
Those are mixed well and used for cleaning purpose as it is an oxidizing agent so it should be handled with extra care.
Different type of glassware's :
1.Pipette : it is a narrow glass tube with both ends open for transferring and measuring liquids by sucking them into the tube. The tip of the pipette is usually tapered.
Types : usually there are two types:
a. Graduated pipette.
b. Volumetric pipette.
Special type:a.Micro pipette or capillary pipette.
b.Van-slake pipette.
c.Automated pipette.
Usual size : 1ml, 2ml, 5ml, 10ml.
Use : For measurement of fluid, Reagent, Blood, Serum
2.Barrette : Barrette is a special hallow glass tube usually with a stopcock at the lower end. It is marked so that the amount of liquid released through the outlet will be known.
Usual size : 5-50 ml capacity. Micro Barrette have a maximum capacity of 2ml or less.
Use : a.in titration.
b.Any volume of liquid up to a certain limit may be out taken out with great accuracy.
3.Measuring cylinder : it is a long cylindrical piece of glass ware with calibration marking on it in on ascending order (zero at the button) a lip to facility pourcing liquid.
Usual size : measurement of relatively large volumes of liquid’s when accuracy is not essential.
Use : measurement of relatively large volumes of liquids when accuracy is not essential.
4.Flasks : a flask is a small bottle with a narrow neck.
Type :a.Volumetric flask
b.Conical flask
c.Rounded bottom flask
d.Flat bottom rounded
a.Volumetric flask : it is a pear-shaped, flat bottomed bottle made of high quality glass. It has a narrow long neck filled with ground glass and a single calibration mark.
Usual size : 50ml, 100ml, 500ml and 1000ml capacity.
Use : For titration
For boiling solutions when it is necessary to give evaporation to a minimum.
5.Beaker: beaker is a glass vessel with wide mouth. It is usually made of heat resistance glass.
Usual size : 5-500ml
Use : a.For weighting reagents in the preparation of solution.
b.For boiling of water and liquid.
c.Graduated beakers is used for estimating the volume of fluid.
6.Funnel : it is a conical, wide-open mouthed device for pouring through its open tube at end into another vessel.
Type : a.Filter funnel.
b.Separating funnel.
c.Filter funnel : it has a conical head and long narrow tube.
Use : to pour liquid’s into container.o To support filter paper during filtration.
b.Separating funnel : it is a special funnel, usually pear shaped or cylindrical. It has fitted ground glass stopper at the top and a plastic stop cock at the button.
Use : To separate two immiscible fluids. Eg, ether and water.
7.Reagent bottle : it is a glass container, has many shapes the usual shape is cylindrical with a narrow neck. It may be clean or coloured and upper end fitted with ground glass.
Usual size : 25-1000ml.
Use : for holding reagent, solution’s, specimen and media.
8.Test tubes : these are tube link containers with one open end.
Usual size : a.6 inch x 5/8 inch
b.20 ml capacity
Use :a.To carry out tests like biochemical, serological.
b.Test tubs are used to heat liquid.
c.Preserve sample like blood, urine, CSF etc.
9.Test tube holder : it has a catch and a handle.
Use : to hold test tube during heating.
10.Test tube rack : these are made of metal, plastic.
Use : to hold the test tube in upright position.
11.Syringe : a.Syringe has the following part- barel is provided with a needle.
b.Plunger which can be moved forwards and back wards.
Usual size : 3ml, 5ml, 10ml, 20ml and 50ml.
Use : to draw blood from blood easels.
To inject drugs.
12.Evaporating basin : it is an open, bowl like shallow container made of porcelain or heat resistant glass.
Use : to evaporate solutions to dryness.
13.Gas burner : to health solution, reagent etc.
14.Barrette stand : to hold barette.
Different types of laboratory equipments (apparatus) :
1.centrifuge Machine:
principal : when centrifugal force exceeds the force of gravity the heavier substances are thrown to the bottom of the centrifuge tube.
Use : to hasten the deposition of substance suspended in a liquid. The suspended matter are deposed, in order of weight, the heaviest one is settled first.
2.photoelectric calorimeter : it is an instrument that measure the intensity of color of a solution by registering the amount of a light observed by a given solution.
Parts : this instrument consists of –
a.source of light.
b.Monochromatic light and filter.
c.Curettage or tubes.
d.Photosensitive elements.
e.A sensitive galvanometer.
Calorimeter is based upon two law –
a.Lambert law
b.beer’s law.
Use : a.to measure concentration of substances such as glucose, cholesterol, electrolytes (na+, k+, ca+), enzymes (ast, alt), haemoglobin etc.
b.to measure activity of enzyme.
PREPARATION OF 100ml NORMAL SALINE.
Principle : Normal saline means 0.9gm% Nacl that is when 0.9gm of Nacl will present in 100ml of solution.
Ingredients :
a.0.9gm Nacl
b.100ml distilled water.
Apparatus :
1. Distal balance.
2. Beaker.
3. Glass rod, Stirrer.
4. Funnel.
5. Volumetric flask.
Procedure :
1. Distal balance is adjusted.
2. 0.9gm of NACL is measured by the balance.
3. then the salt is transferred to a beaker and few ml of water is added to it.
4. it is mixed well with the help of stirrer.
5. then it is transferred into the volumetric flask with the help of funnel.6. lastly water is added up to 100ml marked and shacked well. Thus 100 ml of normal saline is prepared.
Precaution :
1. Weight should be take properly.
2. Lower end of the meniscus of water should be count.
3. Distilled water should be used.
Comment : Normal saline is an isotonic, so it can be used during dehydration shack burn. It is also used for wound, washing and surgical toiling.
EATIMATION OF BLOOD UREA BY ENZYMATING MATHOD USING UREASE.
Principle : urea is hydrolyzed in the presence of H2o & ureose produce NH3 & CO2.
In a modified breathlot reaction NH4+ reacts with hypochlarite and salceilate to from green dye.
the absorbance increase at 578 nm is proportional to urea concentration of the sample.
Recquirment :
A. appartus :
test tube
corvette
pipette
Micro pipette
Centrifuge mechanic
Calorimeter
Filter
Disposable syringe
Test tube rack
B. regent : reagent , PO4 bffer, NA salicilate, Na nitropruslde, EDTA, Regent II, hypochorite,
Standard : urea standard
conc : 80 mg/dl
Enzyme : urease
Reagent preparation : Reagent 1a is prepared by mixing the containt of the bottle enzyme with bottle reagent 1,
1ml enzyme (urease) dissolving 100ml reagent 1, reagent II & standerd are readity for use.
sample : serum
procedure : arranged there cuvette & marked them sample, reagent blank & standared.
Pipetting sheme
pipette in cuvette sample standared reagent blank
standared - 10ml 1000ml
sample 10ml - 1000ml
reagent blank 1000ml 1000ml 1000ml
Regent II 1000ml 1000ml 1000ml
Mixed well & incubetted for 10 min at 25'C temparature & measure the absorbance of standerd and sample againest blank within 60 min.
Assay :
wave length - 578nm
Optical pathway - 1cm
Temparature - 25'C
measurement against reagent blank.
Result :
name of the student : Bishwa
Age : 20
Sex : Male
blood urea level is 22.85mg/dl
Normal level is 10-50mg/dl
Comment : serum urea level is within normal.
Precaution :
1. blood should be drawn with aceptic prceaution.
2. Centrifugation should be completed properly.
3. Appropiate filter should be used.
In a modified breathlot reaction NH4+ reacts with hypochlarite and salceilate to from green dye.
the absorbance increase at 578 nm is proportional to urea concentration of the sample.
Recquirment :
A. appartus :
test tube
corvette
pipette
Micro pipette
Centrifuge mechanic
Calorimeter
Filter
Disposable syringe
Test tube rack
B. regent : reagent , PO4 bffer, NA salicilate, Na nitropruslde, EDTA, Regent II, hypochorite,
Standard : urea standard
conc : 80 mg/dl
Enzyme : urease
Reagent preparation : Reagent 1a is prepared by mixing the containt of the bottle enzyme with bottle reagent 1,
1ml enzyme (urease) dissolving 100ml reagent 1, reagent II & standerd are readity for use.
sample : serum
procedure : arranged there cuvette & marked them sample, reagent blank & standared.
Pipetting sheme
pipette in cuvette sample standared reagent blank
standared - 10ml 1000ml
sample 10ml - 1000ml
reagent blank 1000ml 1000ml 1000ml
Regent II 1000ml 1000ml 1000ml
Mixed well & incubetted for 10 min at 25'C temparature & measure the absorbance of standerd and sample againest blank within 60 min.
Assay :
wave length - 578nm
Optical pathway - 1cm
Temparature - 25'C
measurement against reagent blank.
Result :
name of the student : Bishwa
Age : 20
Sex : Male
blood urea level is 22.85mg/dl
Normal level is 10-50mg/dl
Comment : serum urea level is within normal.
Precaution :
1. blood should be drawn with aceptic prceaution.
2. Centrifugation should be completed properly.
3. Appropiate filter should be used.
DETERMINATION OF SERUM GLUTAMATE OXALO TRANSMILASE BY RATHMAN & FRANKLE METHOD.
Principle: SGOT converts aspartate & ketogluterate to oxaloacetate &glutamate. The form oxaloacetate reacts with 2,4-dinitrophenyle hydrazine to produce 2,4-dinitrophonyle hydrazine which in alkaline media produces from color complex.
Aspartate + ketogluterate SGOT oxaloacetate + glutamate.
Oxaloacetate + 2,4-dinitrophenyle hydrazine--> 2,4-dinitrophenyle hydrazine.
Method: reitmen & frankles method.
Requirements:
A. Apparatus: test tube
Cuvette
Pipette
Micropipette
Centrifuge mechine
Colorimeter
Blood taurniculate
Disposable syringe
Filter.
b. Reagent: reagent I (SGOT substrate)
Composition – P04 buffer
Aspartate
Ketogluterate
Reagent III (color reagent) 2,4-dinitrophenyl hydrazine
Reagent IV: (standard) pyruvate, Na0H
Sample: serum
Procedure: arranged six cuvette & marked them 1,2,3,4,5,6
Addition sequence 1 2 3 4 5 6
enzgnatic activity 0 22 55 95 150 215
Distilled water 100ml 100ml 100ml 100ml 100ml 100ml
Reagent I 500ml 450ml 400ml 350ml 300ml 250ml
Reagent IV 0 50ml 100ml 150ml 200ml 250ml
Reagent III 500 500 500 500 500 500
Mixed well allowed to stand at room temparature for 20 minutes.
1 2 3 4 5 6
Na0H 5000 5000 5000 5000 5000 5000
Ploted earlier
Assay:
web length : 505nm
optical pathway : 1cm
Temparature : 37oC at room Temparature measured against distilled water.
Result :
Name of student : Mahbub alam
age : 20
sex : male
ast level is : 500ml
Normal level is >40 u/ml
Comment: ast level is above normal.
Precation:
1. Blood should be drawn with aceptic precaution.
2. Centrifugation should be completed properl.
3. Appropriate filter should be used.
Aspartate + ketogluterate SGOT oxaloacetate + glutamate.
Oxaloacetate + 2,4-dinitrophenyle hydrazine--> 2,4-dinitrophenyle hydrazine.
Method: reitmen & frankles method.
Requirements:
A. Apparatus: test tube
Cuvette
Pipette
Micropipette
Centrifuge mechine
Colorimeter
Blood taurniculate
Disposable syringe
Filter.
b. Reagent: reagent I (SGOT substrate)
Composition – P04 buffer
Aspartate
Ketogluterate
Reagent III (color reagent) 2,4-dinitrophenyl hydrazine
Reagent IV: (standard) pyruvate, Na0H
Sample: serum
Procedure: arranged six cuvette & marked them 1,2,3,4,5,6
Addition sequence 1 2 3 4 5 6
enzgnatic activity 0 22 55 95 150 215
Distilled water 100ml 100ml 100ml 100ml 100ml 100ml
Reagent I 500ml 450ml 400ml 350ml 300ml 250ml
Reagent IV 0 50ml 100ml 150ml 200ml 250ml
Reagent III 500 500 500 500 500 500
Mixed well allowed to stand at room temparature for 20 minutes.
1 2 3 4 5 6
Na0H 5000 5000 5000 5000 5000 5000
Ploted earlier
Assay:
web length : 505nm
optical pathway : 1cm
Temparature : 37oC at room Temparature measured against distilled water.
Result :
Name of student : Mahbub alam
age : 20
sex : male
ast level is : 500ml
Normal level is >40 u/ml
Comment: ast level is above normal.
Precation:
1. Blood should be drawn with aceptic precaution.
2. Centrifugation should be completed properl.
3. Appropriate filter should be used.
